To develop advanced innovative system based on micro-and nano-technologies with the final goal of an early diagnosis, prevention and effective treatment of Diseases in a cost-effective format for the adoption of a patient centric model.
In particular, the project addresses three, main research lines:
- Development of advanced technological solutions for in vitro diagnosis: Biosensor for nucleic acid and proteins;
- Development of “smart” molecular drug delivery systems for targeted and effective pharmacological therapy;
- Development of a specific ICT infrastructure for the direct transmission of clinical data of biosensors and drug delivery systems to electronics health record.
For the proper development of these innovative technologies are identified appropriate clinical application fields, both in diagnosis and therapy. These define the clinical target requirements to develop the research and to validate the final results of the research. These applications are selected based on:
- Clinical relevance of diagnostic/therapeutic targets (clinical utility) unmet by the current detection/drug delivery technologies available on the market (clinical gap);
- Sicilian region specific clinical requirements and industrial peculiarities;
- Relevance of reference markets.
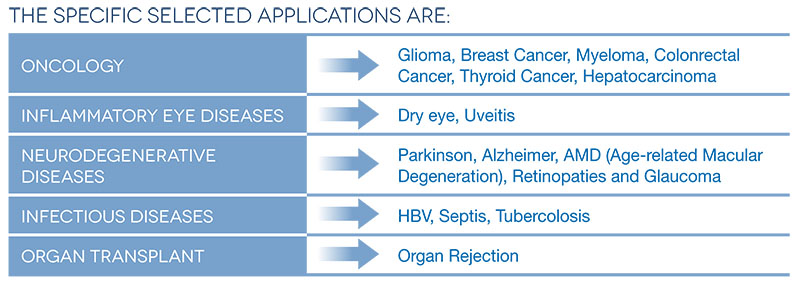
The results of this research will account for the ‘feasibility study’ for the subsequent development of new products in the areas of:
- in vitro diagnostic devices, which in Italy have a market worth about € 1.7 billion;
- ‘intelligent drugs’, with particular emphasis on the ophthalmics, which in Italy have a market worth about 366 million euro;
- system integration for health which is one of the tools that the NHS (National Health Service) plans to implement to contain
- health and social spending, which is expected to increase by 2020 from 13% to 21% of GDP (Gross Domestic Product).
HIPPOCRATES: MAIN WORK PACKAGES
In order to maximize in synergic and effective way the proper contribution to research, after an initial phase of wide technology exploration (exploration phase), there will be a selection of methods/technologies that will show the better performances according to the criteria and quantitative parameters established within the design specifications. These methods/technologies will go to the next phase (engineering and testing phase) aimed to develop the final prototype demonstrators that will be clinically verified in the final stage (validation phase).
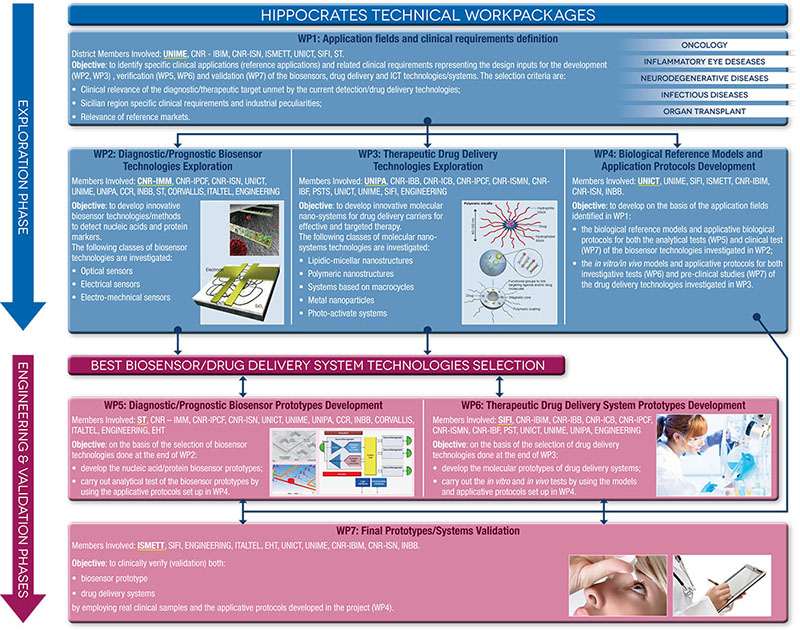
The main deliverables are:
- Biosensor prototypes that allow in a cost-effective format and easy to se way sensitive nucleic acids and protein markers detection;
- Drug delivery systems for a more effective and targeted therapy reducing the drug toxicity and improving the patient compliance;
- ICT infrastructures for direct sending of the clinical data to electronic health record.



